Impact of Social Heterogeneities in Epidemic Outbreaks
Compartmental models are very successful modeling paradigms in epidemiology. Typically, they are used for quantitative assessments of key parameters such as the basic reproduction number. These models rest on two key assumptions: That a population is well mixed and that transmission is triggered by a population-averaged contact rate.However, experimental evidence shows that contact rates vary substantially, and it has been shown that this variability can change the dynamics of a disease. The most important example being the epidemic of sexual diseases. Their spreading would not be possible without the large variation in the number of sexual partners across the population.
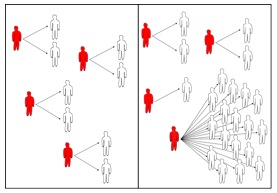
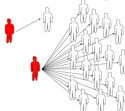
Figure 1: Secondary cases
are infections caused by an infectious individual.
Typical cases in a homogeneous populations (left) and
in a heterogeneous population, where the number of
secondary cases can vary extremely (right).
The SARS epidemic of 2003 highlighted the necessity of a better understanding of social heterogeneities and individual-level based models. So called superspreaders – individuals with a large share of caused infections – were crucial for the spreading of the disease.
We are trying to asses the impact of social heterogeneities in spreading and prevalence of infectious diseases using different models for epidemic outbreaks.
Since disease transmission is a probabilistic process, we would like to understand the influence of heterogeneity in contact rates on the fluctuations of the process, which can lead an epidemic to extinction or to a larger number of infected individuals.
We are also interested in the impact of social heterogeneities in the estimation of the basic reproduction number – the average number of secondary infections caused by one infected individual – since predictions for the outcomes of epidemic outbreaks rely on the estimation of this parameter.
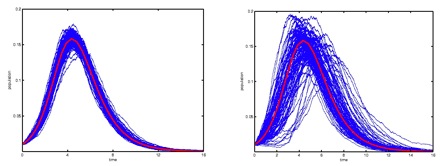
Figure 2: Number of
infectious over time in homogeneous (left) vs,
heterogeneous populations (right). Among the
differences we observe, epidemics become rarer but more
explosive, time delays appear which may cause epidemic
outbreaks to develop without notice, and the
uncertainty of predictions grows as a populations
become more heterogeneous.
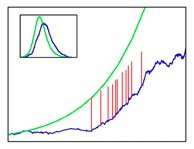
Figure 3: Superspreading
events: Vertical red lines show infections caused by
superspreaders in a typical simulation (the plot shows
number of infectious over time). The presence of these
individuals can drastically alter the course of an
epidemic outbreak.
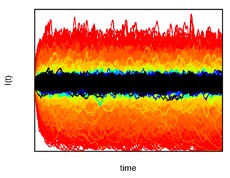
Figure 4: Fluctuations
around the endemic state. In homogeneous populations,
certain diseases' prevalence fluctuates around an
endemic state (fixed point). Here, we show that as the
variability in contact rates diverges in heterogeneous
populations, the fluctuations around the endemic state
increase.